|
Live-Cell
Imaging:
2. Near-Field
Optical Microscopy (NSOM)
We have built a custom designed NSOM system
that employs a fiber-optic based cantilever tip that allows
simultaneous probing of surface morphology using tapping-mode
atomic force microscopy (see schematic below)
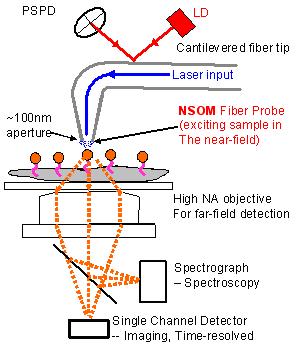
Figure 1. Schematic of combined atomic force microscopy and
near-field scanning optical microscopy
I. Introduction to AFM/NSOM Instrumentation
II. AFM/NSOM Imaging of red blood cells
III. AFM/NSOM Imaging of cancer cells
I. Introduction
to AFM/NSOM Instrumentation
To complement studies using conventional
optical microscopy techniques and to obtain topographic and
optical images of cells with nanoscale resolution, we developed
and employ a unique optical microscopy setup that combines
tapping mode atomic force microscopy (AFM) and near-field
scanning optical microscopy (NSOM) as schematically shown
in figure 1.
The setup works in a manner similar
to a normal force tapping mode AFM, except that the conventional
tip is replaced by a tapered optical fiber with sub-wavelength
aperture ~100nm in diameter at the end. Light excitation is
delivered through the fiber. The diffraction through the subwavelength
aperture creates a near field at the end of tip which excites
the almost only fluorofores on the surface of the cell. Therefore
simultaneously 3D morphological information and surface sensitive
fluorescence imaging can be obtained. Since the resolution
the NSOM is decided by the diameter of the aperture, the diffraction
limit of optical microscopy is broken. Nanoscale distribution
of molecules thus can be obtained.
Below we show a couple illustrative examples of cell image
using AFM/NSOM.
II. Imaging
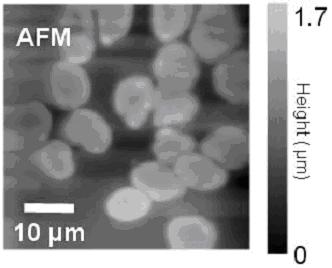
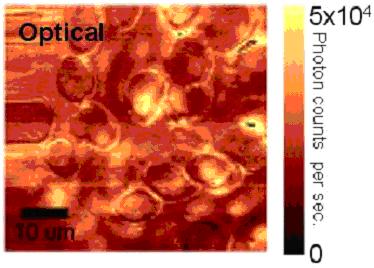
of red blood cells
The optical and topographic images obtained simultaneously
for red blood cells are shown below:
III. Imaging
of cancer cells
Applications of quantum dots as biological
probes in conjunction with nano-scale detection can be extended
towards other novel and medically significant uses such as
the early detection of cancer which is currently another area
of our focus. We are working in collaboration with Prof. Richard
Cote, Dr. Ram Dattar and Dr. Deborah Hawes's laboratory in
the department of Pathology at the USC Health Sciences Campus
to work towards establishing new paradigms that would enable
early detection of cancer through the use of Nanotechnology.
Our approach is to obtain simultaneous topographical and optical
information from cancerous versus normal cells using the near-field
optical microscope to be able to distinguish any structural
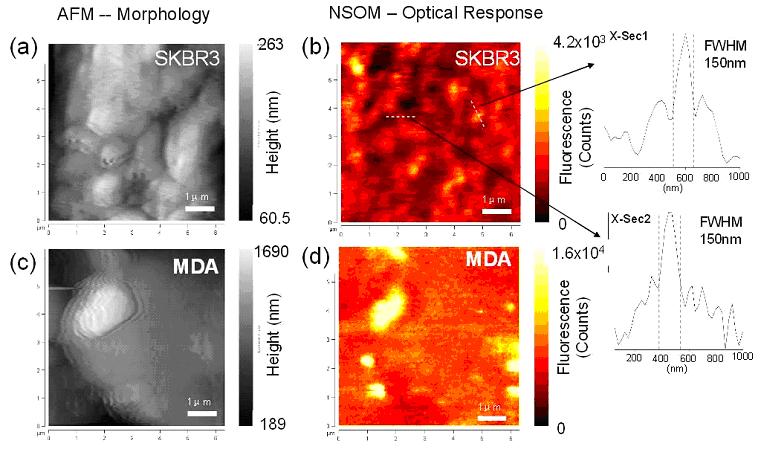
and/or optical differences at the nano-scale. The figure below
shows simultaneously obtained topographic and fluorescence
optical images of breast cancer cells SK-BR-3 and MDA-MB-231.
The cells are labeled with CdSe/ZnS (600nm emission) quantum
dot targeting Her2/neu receptors on the cell surface. Images
are obtained using a tip of 100nm diameter aperture. The resolution
of the NSOM images, as indicated by the smallest features
on it, is ~150nm. Note that the fluorescence NSOM image of
both SKBR3 and MDA cell is marked by bright areas of ~500nm
diameter. This indicates Her2/neu receptors on the SKBR3 cell
surface are not distributed evenly but instead localized in
clusters. . The only difference is that the number of such
clusters on MDA is much smaller than on SKBR3 cell, which
is understandable given the total lower number of Her2/neu
receptor on MDA (2x104/cell) than on SKBR3 (1x106/cell).
Back to
Live-Cell Imaging Menu
|